What is a Batch Record?
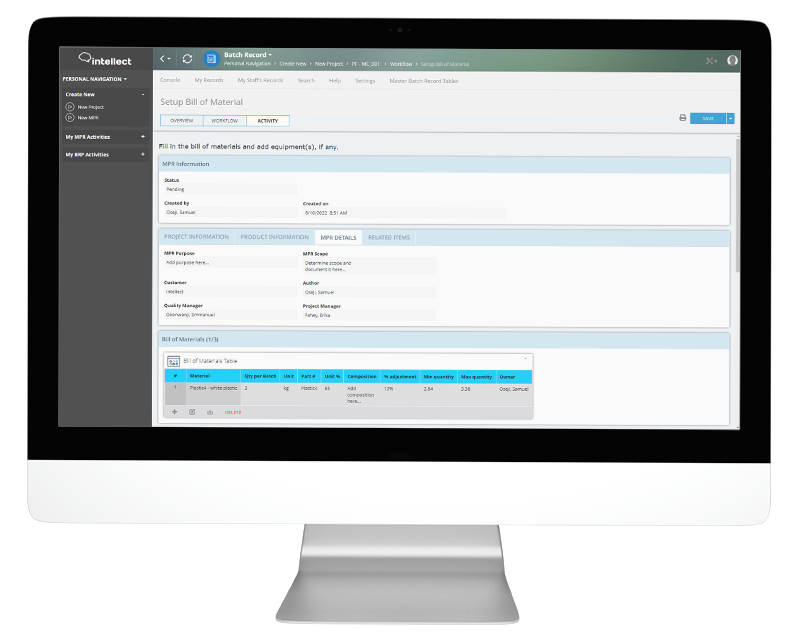
A batch record documents the history of a manufactured product. It includes processing instructions to ensure its safety, quality, and conformance to GMPs. Batch records also contain specific information about product tests including analytical methods used and test results.
The FDA requires a different Master Production Records for each formulation and batch size, which can be complicated to manage. Using electronic batch records simplifies this process and reduces human error.