Avoid Human Error and Improve Quality
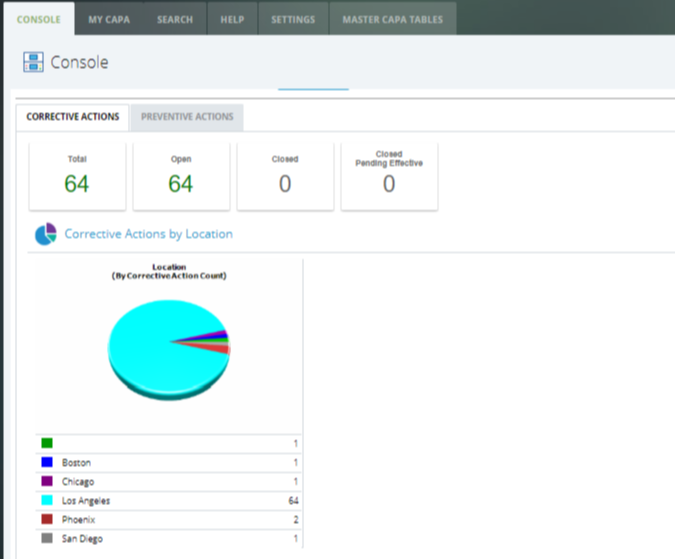
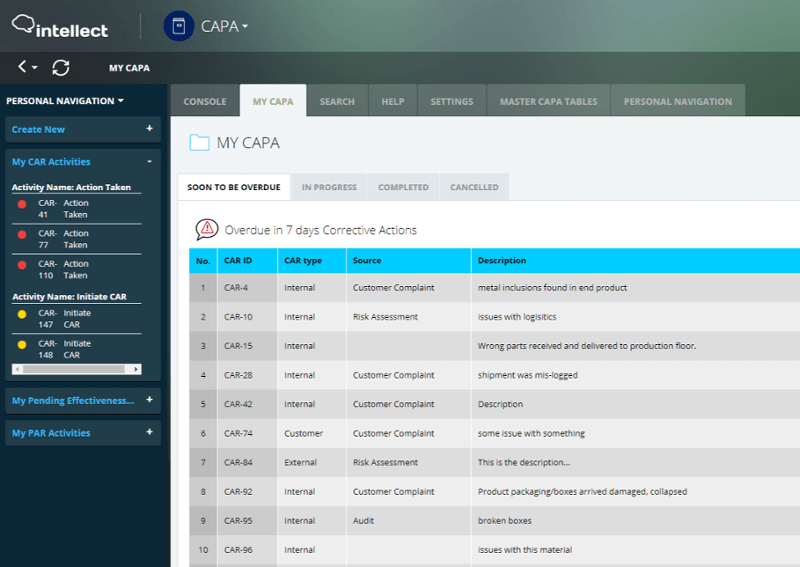
Manual systems leveraging excel or other homegrown solutions are inefficient, prone to human errors, and may delay the resolution to an incident. Intellect’s CAPA application automates and streamlines the entire process, from data collection, follow-up, escalation, and resolution of existing or potential nonconformances.
Users know when and what to do and can review tasks or complete approvals on any device, at any time. At the same time system overcomes bottlenecks, send automated email notifications and reminders that drive accountability and task completion.
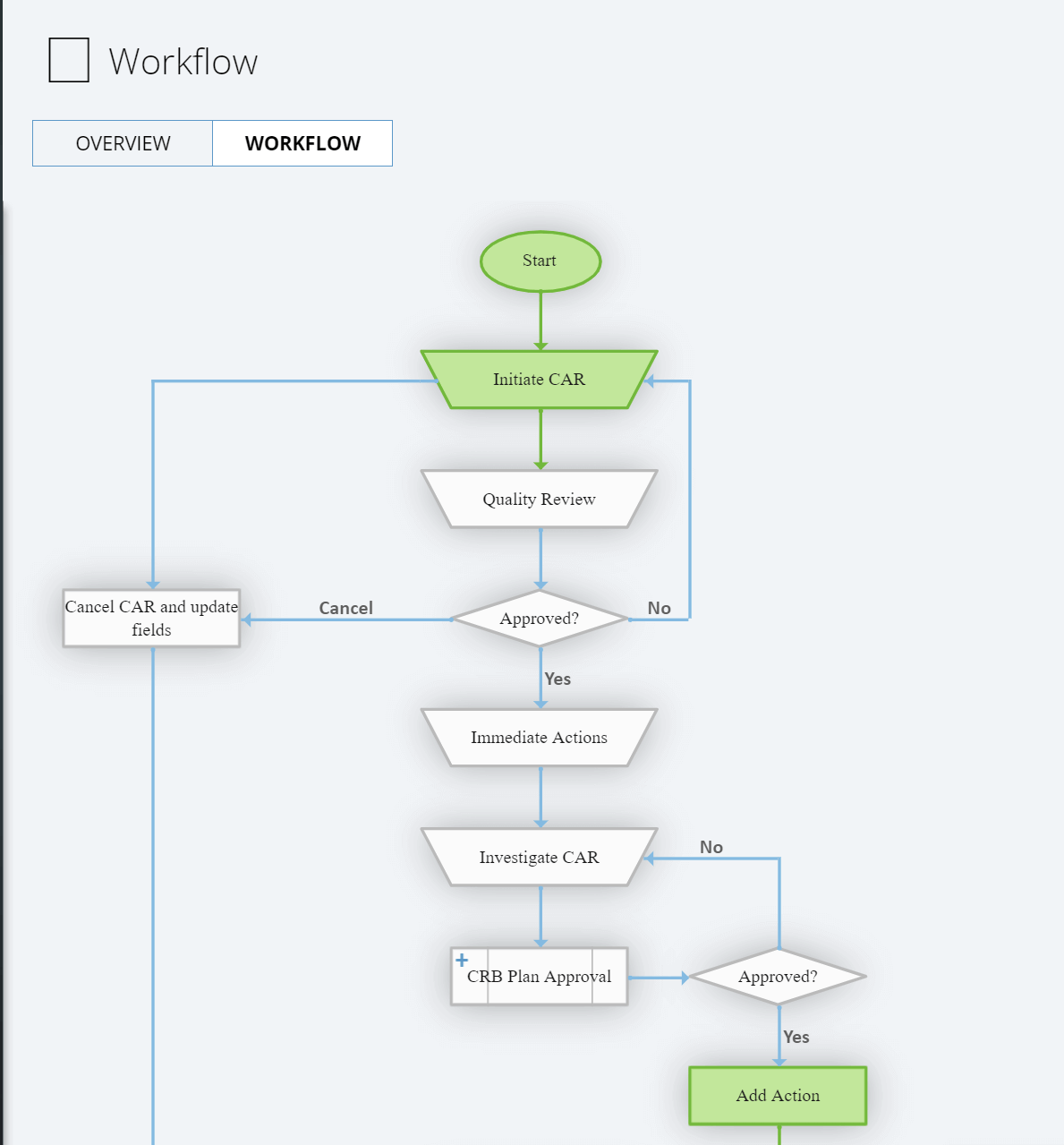
Intellect offers a 8D problem solving methodology to facilitate continuous improvement through identifying, classifying, and eliminating existing or potential nonconformances in product, processes, and system.