Truly Versatile
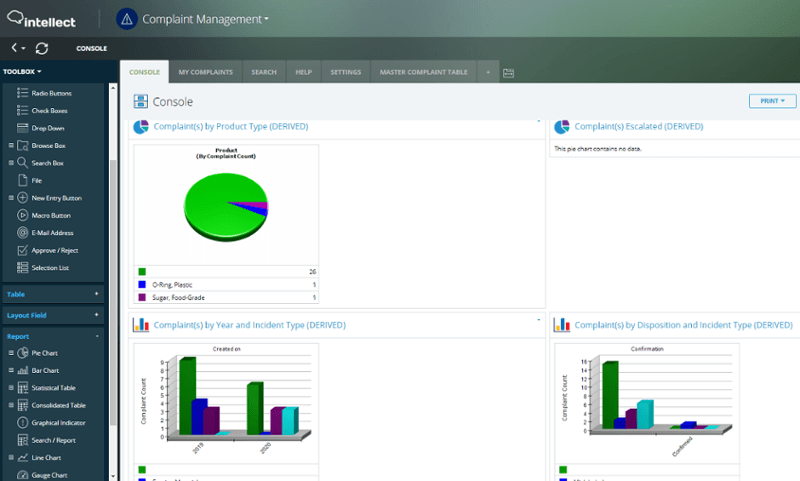
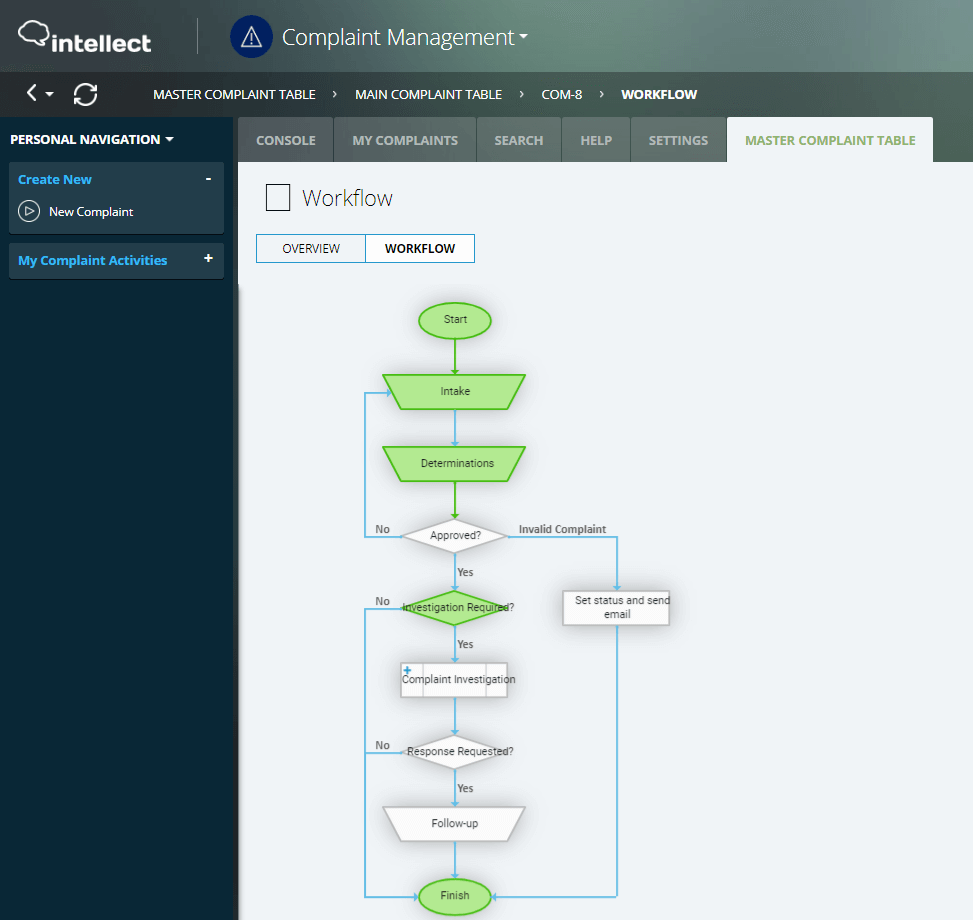
Quality, compliance, safety, risk, efficiency, and costs are impacted in addition to poor customer communication without a proper Customer Complaints Management software system in place. Intellect QMS includes change management, automation, version control, and workflows to communicate, acknowledge, track, and then implement any requirements.
Our solution increases customer success, improves product quality and safety, reduces product recalls and ensures compliance. The ability to integrate with over 500 systems and highly customizable workflows provides unlimited versatility.