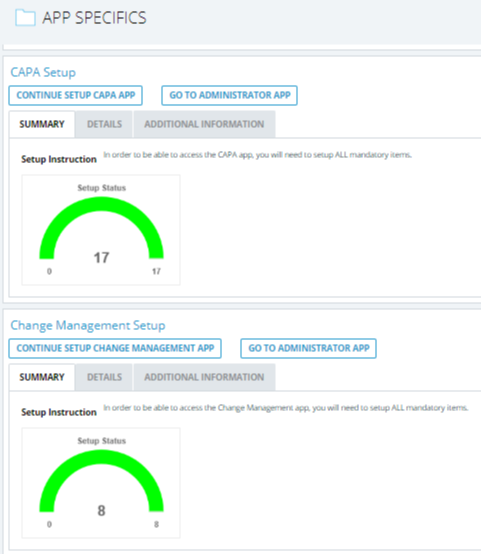
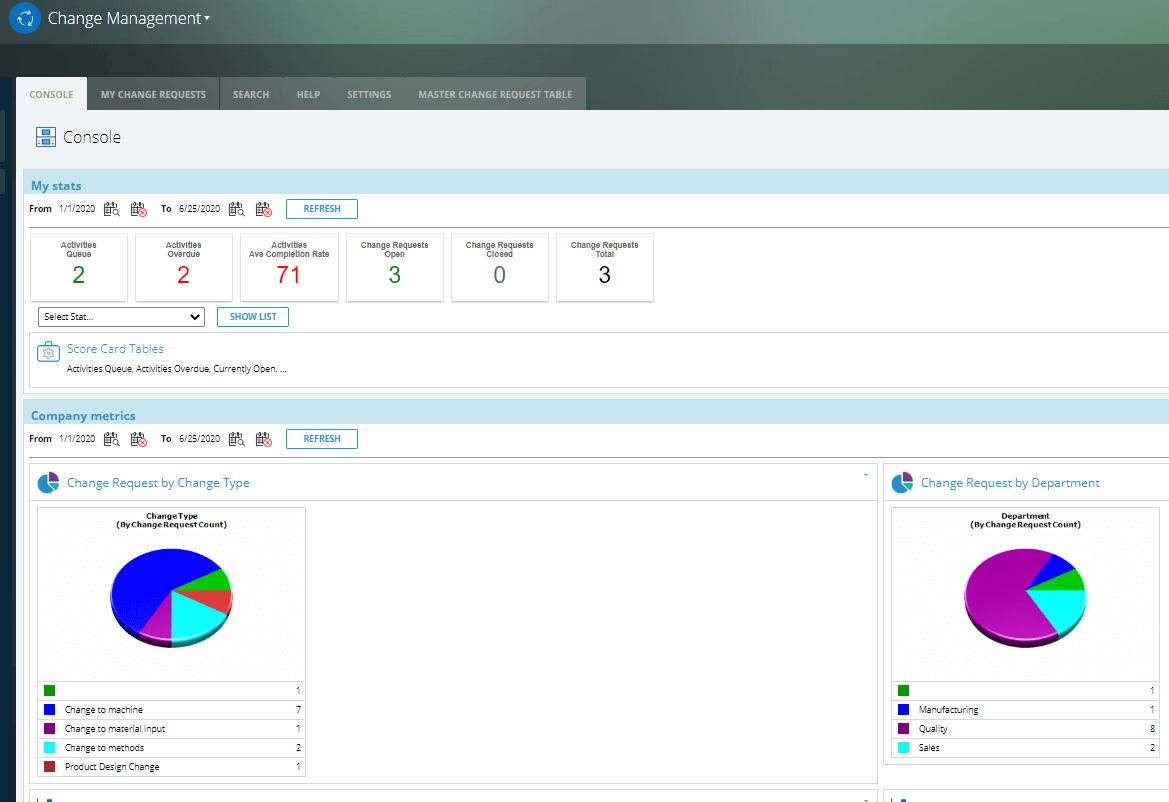
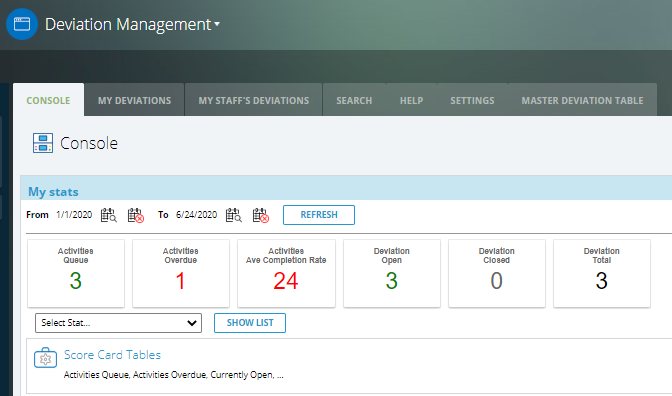
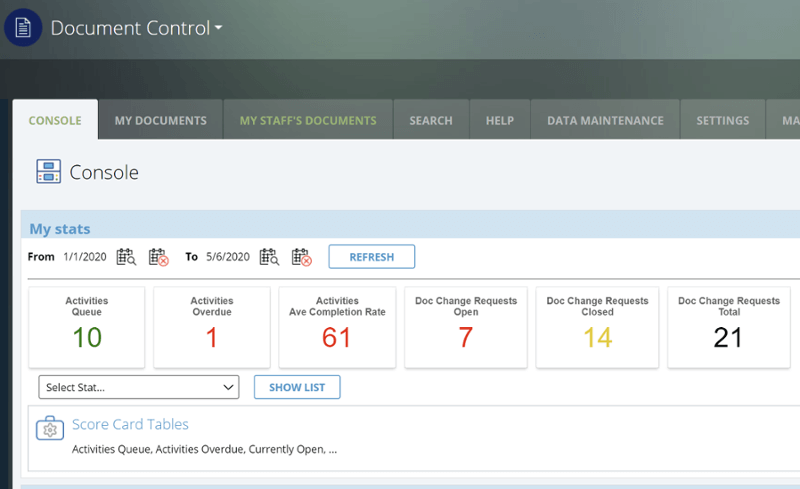
CAPA
✓ 8D problem solving methodology used to facilitate continuous improvement, through identifying, classifying and eliminating existing or potential nonconformances in product, processes, and system
✓ Establish the mechanism to perform of finding the root cause of a problem, devise a short-term fix and implementing a long-term solution to prevent recurring problems
✓ Establish CAPA Review Board team to review and approve corrective actions
✓ Reduce the risk of lost data and repeat issues by ensuring that defined CAPA solutions and processes are met.
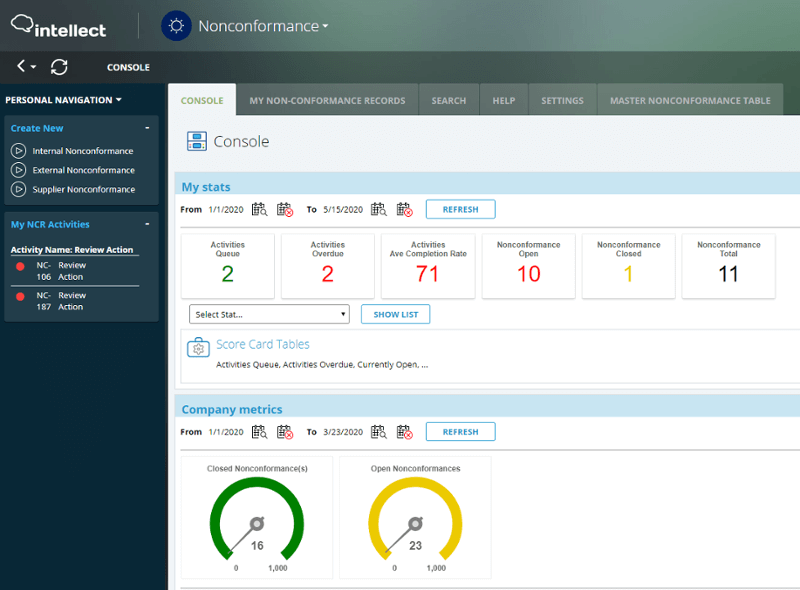
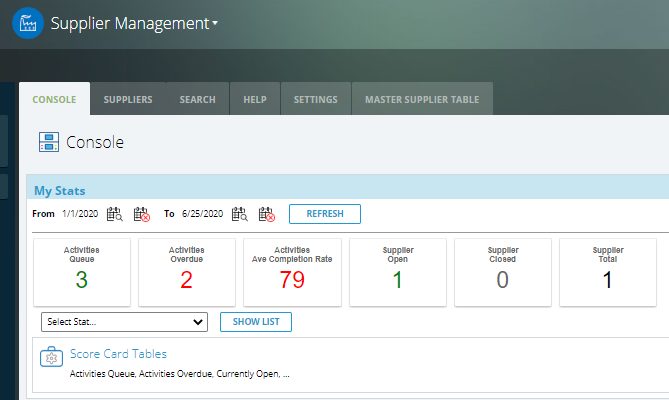
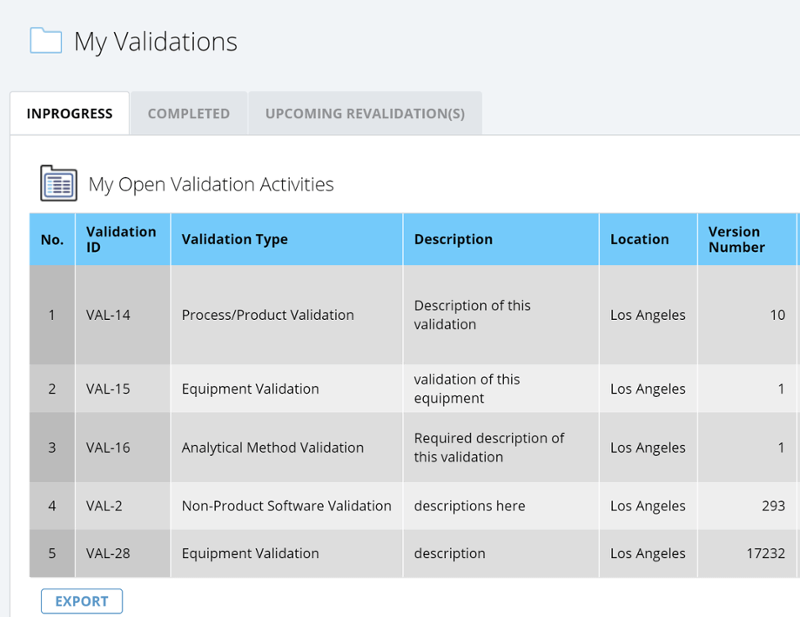
✓ Customizable analytics and reporting to understand trends and solve problems, improve processes, and implement new preventative measures.